Background:
In molecular communication (MC), information is transmitted using molecules. Within the human body, natural MC facilitates essential biological processes such as cellular signaling and neuronal communication. Synthetic MC builds upon these natural mechanisms by employing engineered systems, e.g., for medical applications, including early cancer detection, targeted drug delivery, and health monitoring.
For early cancer detection in the bloodstream, one promising strategy is to monitor cancer-specific biomarkers such as proteases, which are enzymes responsible for protein degradation. In fact, cancer cells often exhibit altered protease expression profiles, with certain proteases contributing to tumor growth, invasion, and metastasis. Therefore, these enzymes are valuable proxies for cancer detection. Among proteases, caspases represent a key subgroup that plays a crucial role in apoptosis, i.e., programmed cell death triggered by cellular damage. Moreover, caspases can be either tumor-suppressive or tumor-promoting, depending on the tissue microenvironment, making them compelling biomarkers in cancer research.
Methods for Protease Detection:
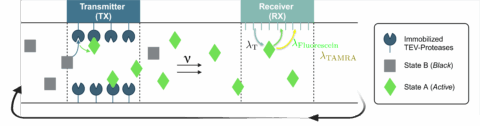
Several approaches have been proposed for detecting the presence of proteases. In [1], the authors demonstrate that two proteases, namely tobacco etch virus (TEV) and caspase-3, can be detected using FlipGFP, a modified variant of green fluorescent protein (GFP). In [2], TEV protease is identified using a fluorescence-quenching-based sensing scheme. In this setup, the fluorescent dyes 5-Fluorescein and Tetramethylrhodamin (TAMRA) are connected through a TEV specific cleavage sequence that contains the recognition motif ENLYFQS, hereby forming the 5-Fluorescein-TAMRA dye. The recognition motif ensures high selectivity because only TEV can process the sequence. When the cleavage sequence is cut by TEV, the fluorescence signal increases. In particular, when the system is irradiated by light with a wavelength at 490 nm, 5-Fluorescein is predominantly excited, while TAMRA is only partially excited [2]. The overall behavior depends on whether the cleavage sequence is intact or already processed.
In the non-cleaved case, i.e., in the absense of TEV, the dyes remain in close spatial proximity. This allows a Förster Resonance Energy Transfer (FRET) process in which TAMRA acts as the acceptor and quenches the fluorescence emission of 5-Fluorescein. The transferred energy is released through non-radiative dissipation, i.e., heat, which results in a low measured fluorescence intensity.
In the presence of TEV, the cleavage sequence is cut, which increases the spatial separation between 5-Fluorescein and TAMRA. The increased distance reduces the efficiency of FRET and therefore decreases the quenching effect. As a result, the measured fluorescence intensity increases.
Overall Project Aim:
In this project, the concept of protease-based early cancer detection in the cardiovascular system in the context of MC will be experimentally verified using a testbed that has been designed and set up by Maike Scherer from the Institute for Bio-Process Engineering (BVT) at FAU. A schematic representation of the working principle of the testbed is shown in Fig. 1.
The main objectives of this thesis can be summarized as follows:
- Get familiar with the working principle of the testbed, including the experimental setup and its operation.
- Conduct a data analysis of existing measurements.
- Develop a simple mathematical model that captures the behavior of the testbed.
- Compare measured received signals to the predictions made in the mathematical model.
Contact Information:
In case you are interested in pursuing this project, please contact Lukas Brand (lukas.brand@fau.de) or Timo Jakumeit (timo.jakumeit@fau.de).
References:
[1] Q. Zhang et al., “Designing a green fluorogenic protease reporter by flipping a beta strand of GFP for imaging apoptosis in animals,” J. Am. Chem. Soc., vol. 141, no. 11, pp. 4526–4530, Mar. 2019.
[2] C. Paththamperuma and R. C. Page, “Fluorescence dequenching assay for the activity of TEV protease,” Anal. Biochem., vol. 659, p. 114954, Dec. 2022.